HPH MRD Personalized Cancer Detection with Ultra-High Sensitivity
HPH MRD is a truly personalized cancer detection test using whole genome sequencing to inform the test design and manufacturing process, delivering an ultra-high sensitivity test for every patient.
HPH MRD is performed in Germany and samples and data remain local.
HPH MRD is based on the technology of Haystack MRD® by Quest Diagnostics®.
MRD Testing Overview
Whole Genome Sequencing Technology
HPH MRD utilizes whole genome sequencing (WGS) technology to identify tumor-specific mutations.
The test is personalized for each patient based on their tumor's unique genetic profile, allowing for highly sensitive detection of ctDNA in blood samples.
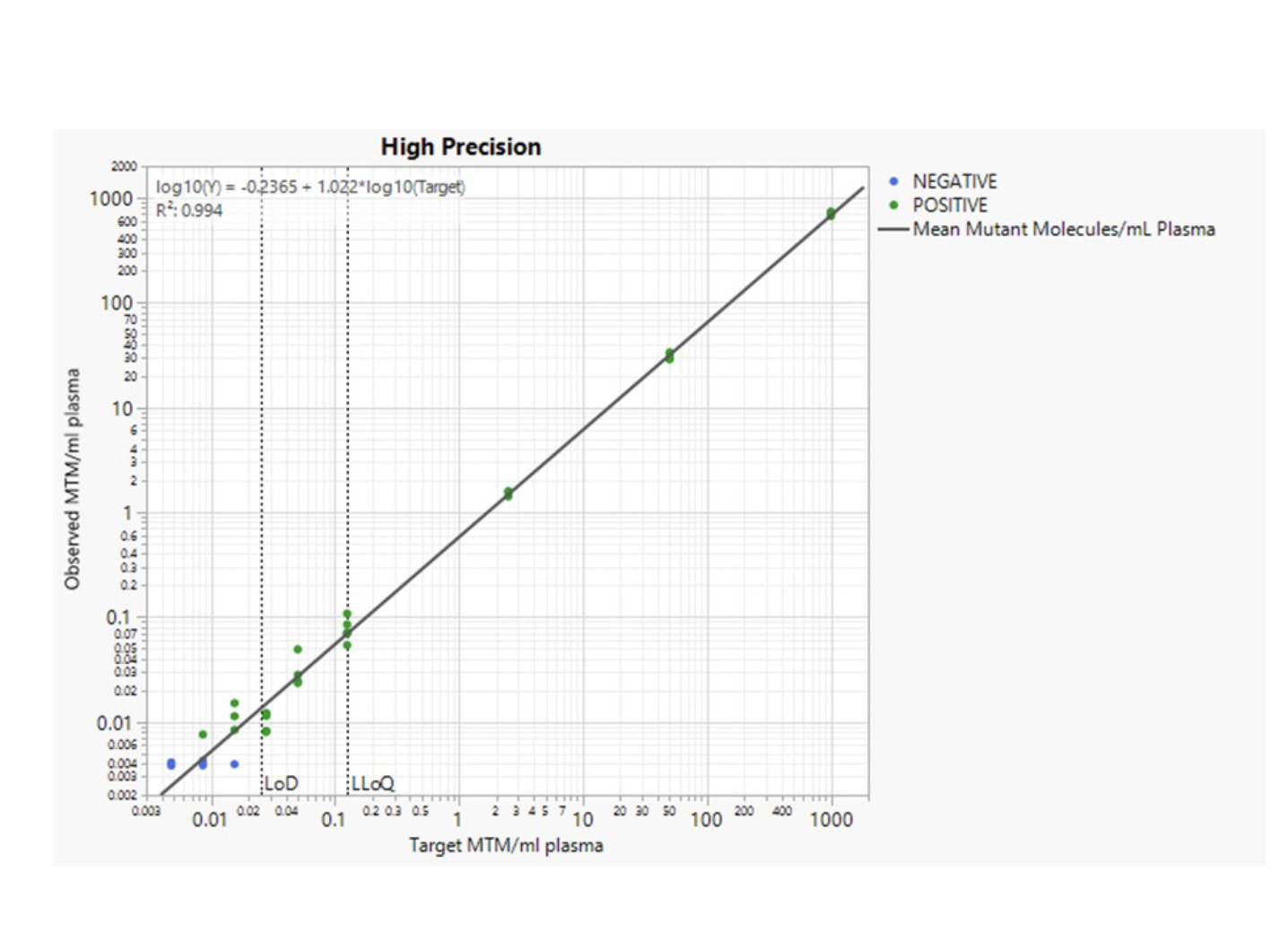
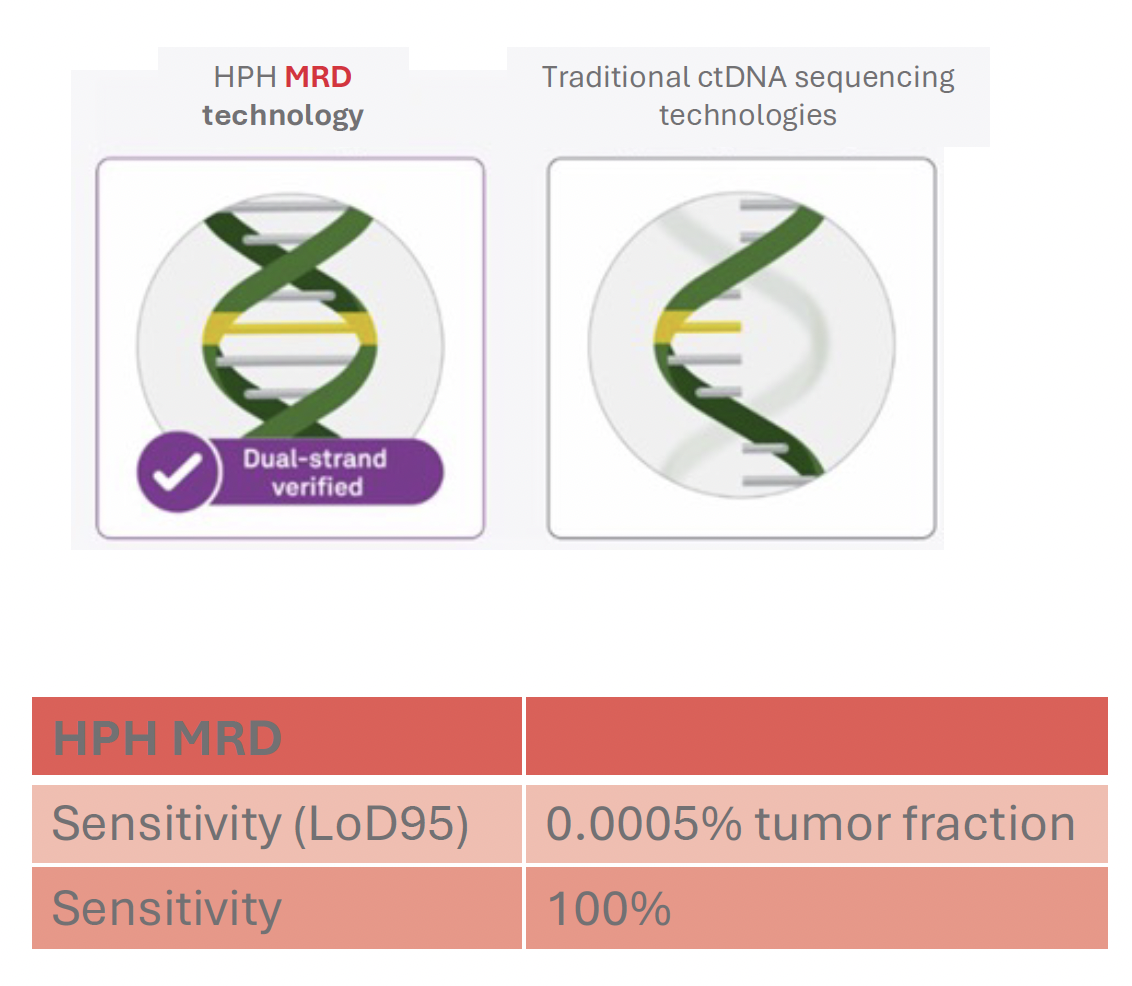
Ultra-High Sensitivity Detection Limits
Circulating tumor DNA (ctDNA) is extracted from a blood draw that contains cell-free DNA next to many other biological molecules.
The main component of cell-free DNA is normal or healthy DNA and only a fraction within this DNA stems from the tumor.
HPH MRD can identify miniscule amounts of tumor DNA in a background of normal DNA and is thus ultra-sensitive.
The limit of detection has been determined to be below 1 ppm (parts-per million), which means that HPH MRD can identify one tumor molecule in one million normal DNA molecules.
This low limit of detection means an ultra-high sensitivity to detect the smallest number of tumor cells in your body.
Baseline and Monitoring Workflow
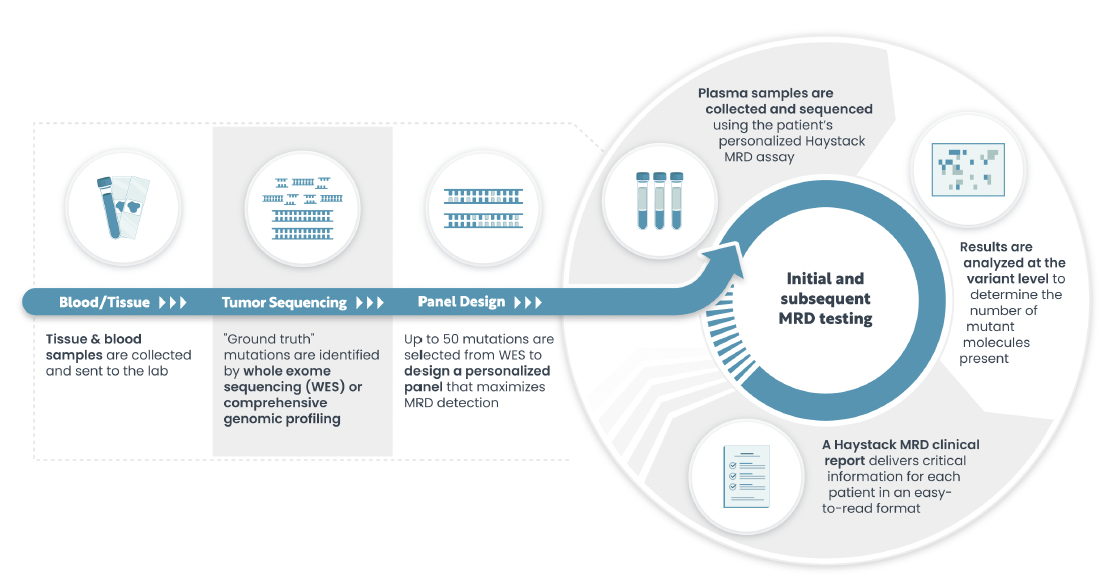
HPH MRD starts with manufacturing of a personalized test based on the genetic fingerprint of your tumor.
To retrieve the genetic fingerprint, we need a small amount of tumor tissue either from the resected tumor or from a biopsy and a blood sample in a specialized test tube (Streck tube).
We will analyze the DNA of both samples and identify the genetic alterations that are specific for your tumor and design a test that can measure these genetic alterations.
This results in the HPH MRD Baseline report.
Follow up testing requires only a blood sample which will be analyzed using the personalized test.
The results will be available in the HPH MRD Monitoring report.
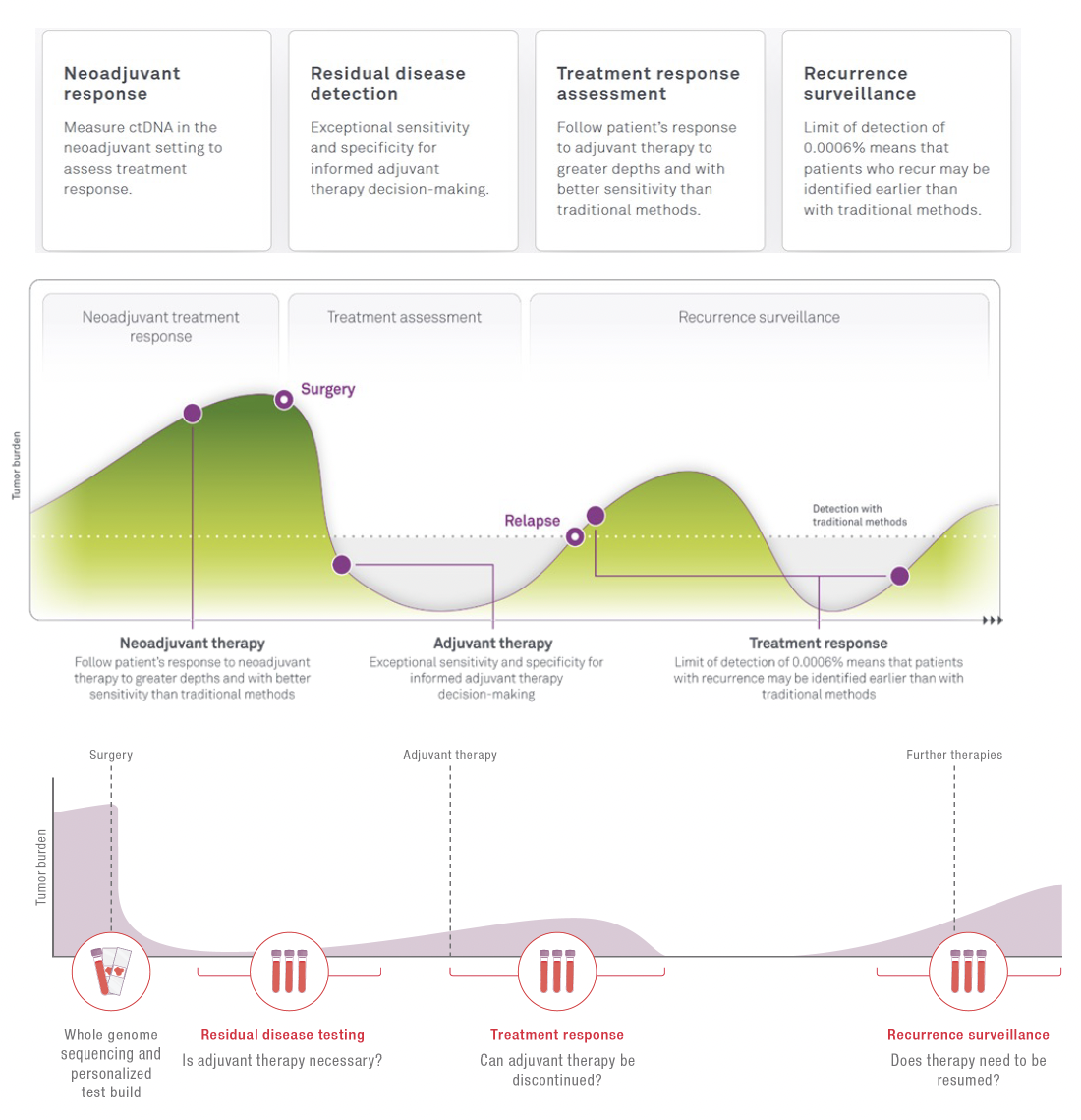
Clinical Use Case Indications

When to use HPH MRD?
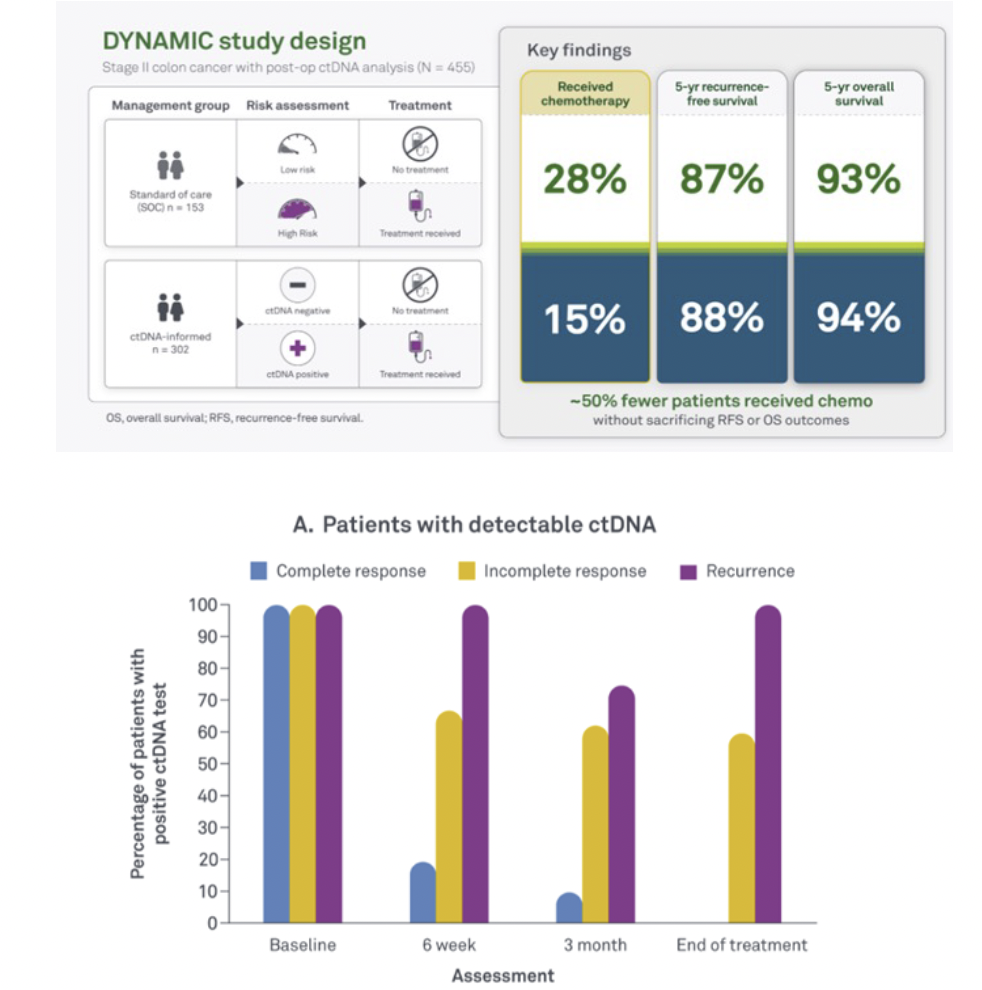
ctDNA is a powerful biomarker and can be measured to assess the effectiveness of treatment at multiple time points in a patient's journey.
Neoadjuvant treatment
Monitor the effectiveness of the treatment and inform the decision for the next steps.
Surveillance
Giving you confidence in your current situation. A simple blood draw at your local physician is sufficient to receive a new result.
Adjuvant treatment
Supporting the decision if adjuvant treatment is needed. Not every patient benefits from chemotherapy or radiotherapy. Treatment with an immune checkpoint inhibitor can be lengthy and you might want to know if cancer can still be detected.
Sample Processing Workflow
HPH MRD requires both a tumor tissue sample and blood samples.
The tumor tissue sample is used to establish the patient-specific mutation profile, while blood samples are used for ongoing MRD monitoring.
Which cancer types can be detected?
HPH MRD detects circulating tumor DNA (ctDNA) from a peripheral venous blood draw and is not specific to any cancer type.
The test can be applied to almost every solid tumor cancer, as long as it sheds DNA into the bloodstream.
The concept of ctDNA detection has been shown for many cancer types, such as colorectal, lung, breast, esophageal, melanoma, pancreatic, bladder cancer and other cancer types.